Modern, Simple and Compliant Document and Learning Management
Say goodbye to paper! Modernize your processes with connected document, training, and controlled Change Management.
Request Demo
With the rise of remote work and global business models you can no longer afford to rely on legacy or paper-based systems to manage your documents and trainings. These systems can easily become a hinderance, slowing down workflows and leading to inconsistencies
Lack of consistent digital content access makes employee skilling and re-skilling to maintain sustainability almost impossible. The never-ending changes to the content only make matters worse.
On the other hand, there is an increase in the number of electronic systems across the organization. Knowledge content, such as policies, procedures, work instructions, job aids, learning videos, regulatory training content, drawings, etc., are authored and maintained, if at all, in many different systems, locations and resources.
At the end of the day, sub-optimal knowledge can lead to employee disengagement and decreased productivity which in turn impacts overall quality, safety and productivity. That’s why it is more crucial than ever to make the right set of content available to the right people at the right time and on any device.
Goodbye Paper and Silos. Hello Digitalization and Productivity.

By implementing a fully connected document and learning management suite of solutions, you give all employees instant access to data anytime and wherever they are, boosting productivity and business agility to new levels.
ComplianceQuest is a cloud-based platform that combines and fully connects Document, Training and Controlled Change Management in the Knowledge Management suite. It enables your teams to:
- Have real-time visibility over content and workforce processes
- Ensure anytime access to the latest version
- Eliminate back-and-forth with easy and compliant online collaboration
- Keep workforce upskilled with on-demand learning
- Always be audit ready
With CQ’s complete Quality management Suite not only can you make every day work easier for your workforce, but you also ensure compliance.
A Modern and Compliant Document and Learning Management System
- Document Management
- Change Management
- SCORM Adapter
- Training Management
To enable your teams compliantly, you need a modern cloud-based system that makes collaboration and knowledge sharing easy and secure.

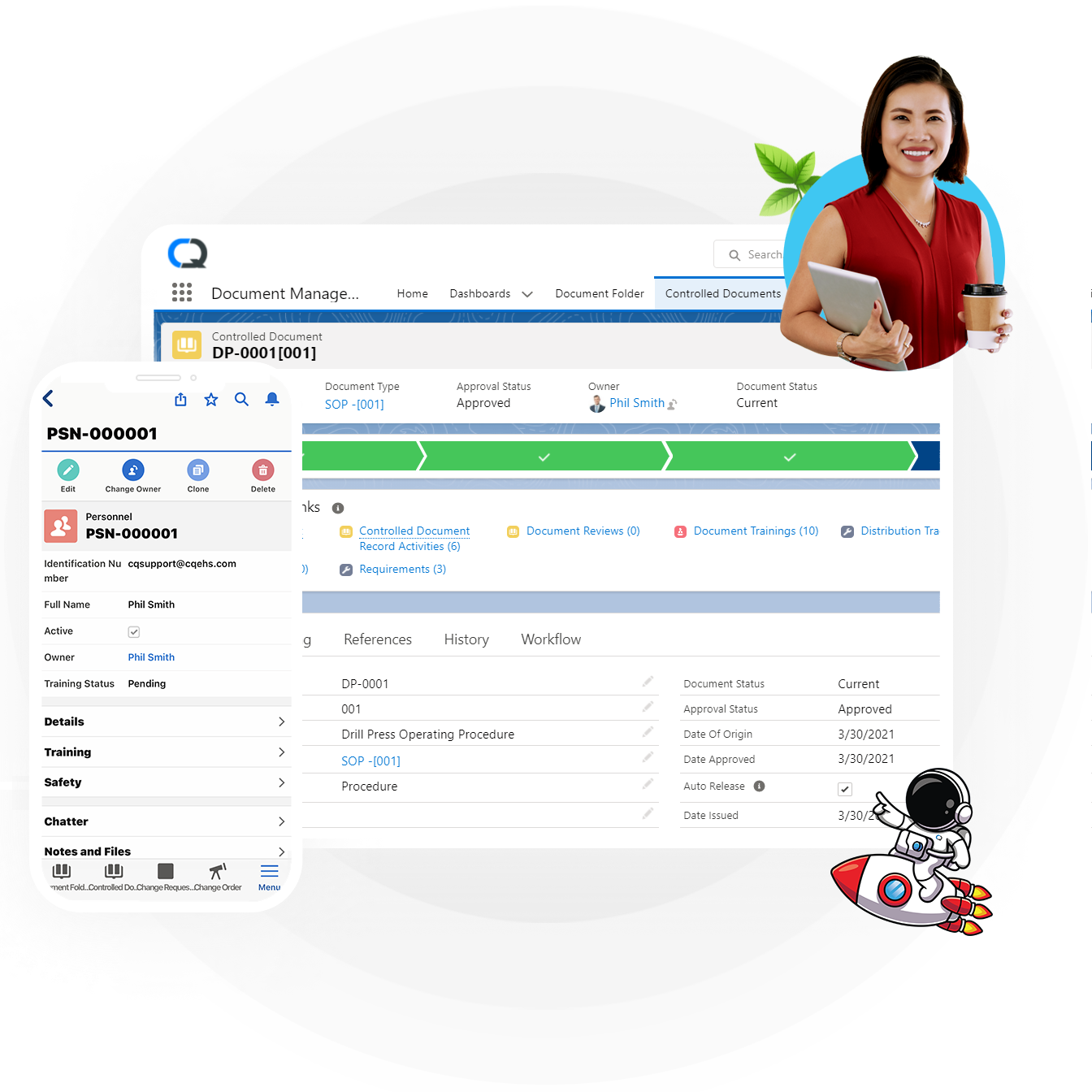
Document Management
Ensure your employees have access to the data they need, when they need it, on whichever device they are using, compliantly. Switch to a unified repository and boost productivity with advanced collaboration tools.
- Automated versioning and approval workflows.
- Integration with Microsoft Office and Google Docs for document collaboration.
- AI powered document search.
Change Management
Ensure compliance by monitoring the execution of all changes and conduct impact assessments to understand organization-wide change effects.
- Documented change workflow tracking and monitoring.
- Collaboration tools with action assigning and tracking.
- Change evaluation and impact assessment.
Training Management
Bridge the skill gap and ensure your employees are always up to speed. Tailor trainings to each employee to upskill your workforce compliantly with a reliable training management process.
- Automated training plans per job description.
- SCORM compliant content.
- Document management integration.

Customer Success
Success Story of 2 Companies Leveraging CQ Document Management Solution to Optimize their Business Processes

Why ComplianceQuest

Beyond Document and Learning Management
-
Quality Management
-
Risk Management
-
Safety Management
Metrics that Matter
-
-80%

Cost of IT Infra Management
-
-45%

CAPA raised per Year
-
-48%

Cost of Quality
-
-50%

Change Request
-
-24%

Cost of Labor
-
+54%

Employee Engagement
-
-32%

Customer Complaint
-
-70%

Employee Training
-
-46%

Audit Finding
-
+37%

Customer Satisfaction
Related Insights
Cost of Quality: Finding the Sweet Spot Between Expense and Excellence
Every company has a key responsibility to take into account the cost of achieving quality, since their goal is to satisfy…
-
Countdown Begins: Preparing for FDA’s Amended Quality Management System Regulations (QMSR) Final Rule
On January 31, 2024, the US Food and Drug Administration…
-
PPAP Management: Streamline Your Product Approval Process Digitally
Optimize and manage your PPAP process with the innovative features…
-
‘Quality Touches Everything’ Series: Infuse Quality into Your Product Lifecycle
In a recent engagement with a medical device company known…