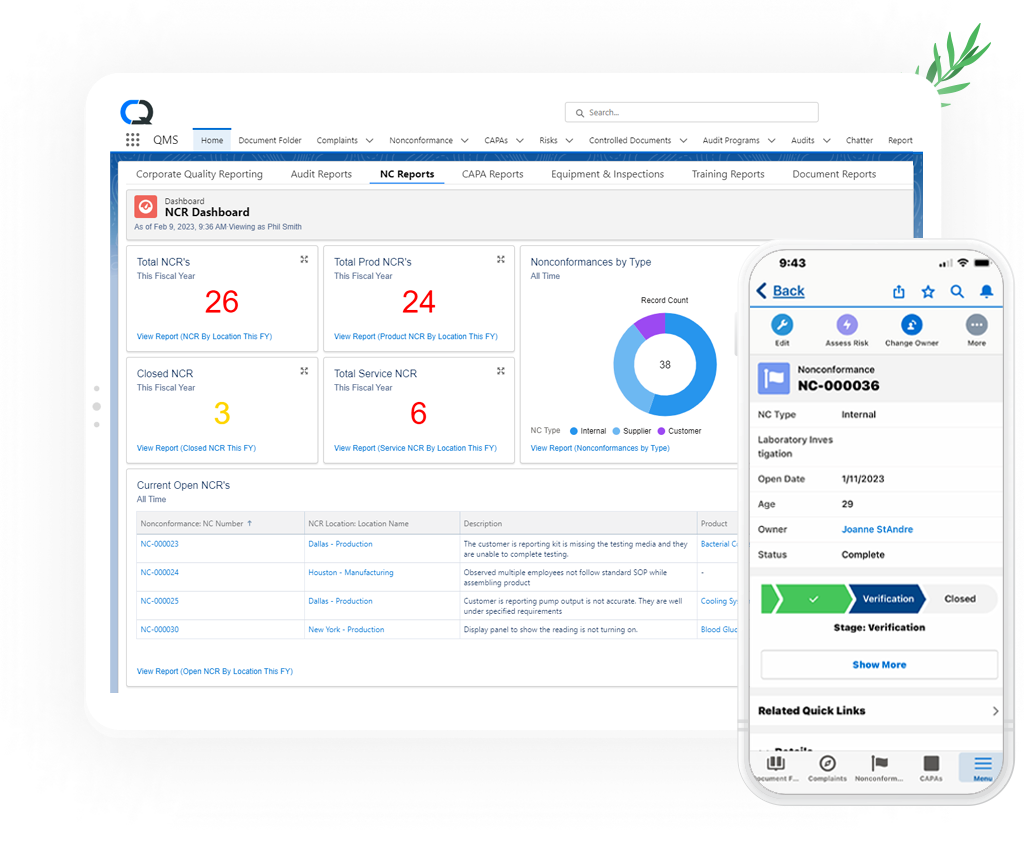
Whatever the source, the CQ Nonconformance Management Solution ensures your teams are capturing, triaging, investigating and closing nonconformances quickly and effectively so you can reduce the cost of poor quality.
Identifying and managing product and process nonconformance and eliminating its cause is critical to an organization’s quality system. This is especially pertinent in the stringent FDA and ISO compliant environments, where a non-conforming product, material, or component could lead to costly rework, or worse, a product recall. Our primary goal, in addition to reducing CoQ, is to capture and act on a nonconformance before it leaves the organization and reaches a customer.